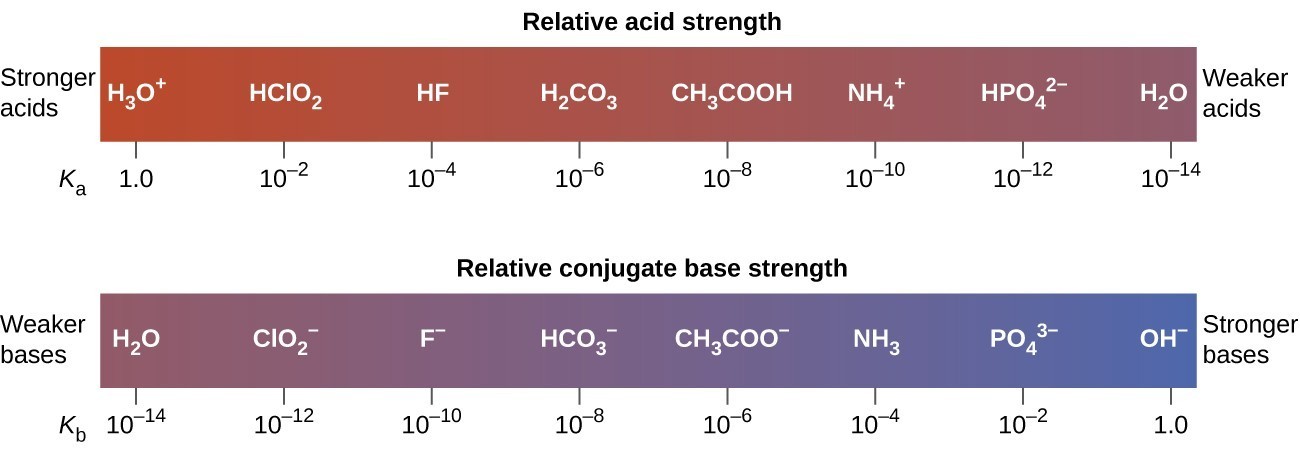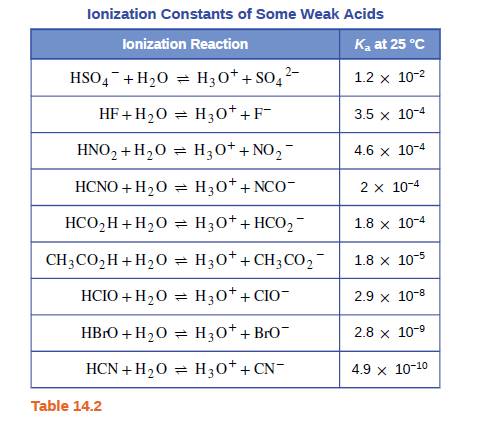
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases. F“ or CN”, is the stronger base? See Table 14.2. | bartleby
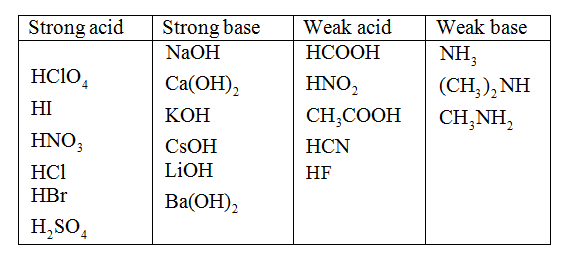
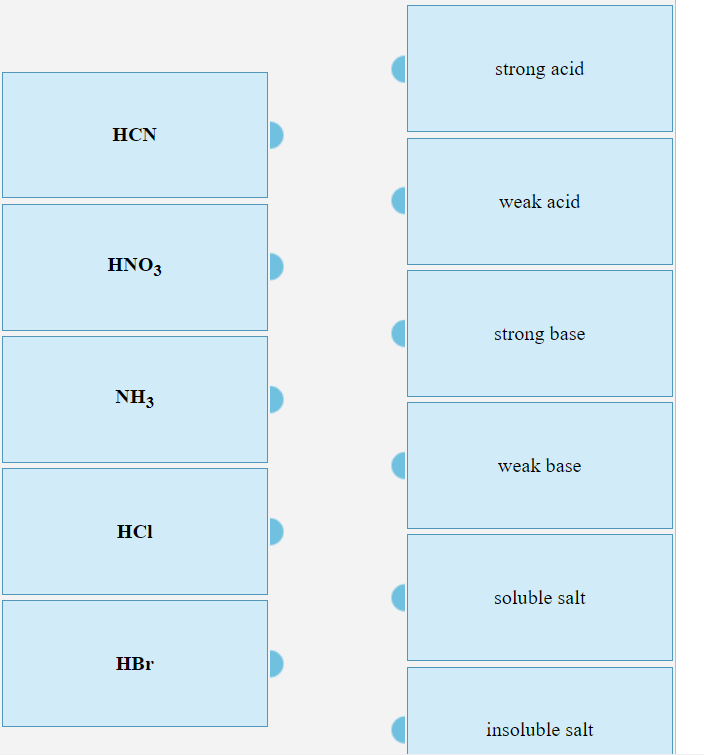
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

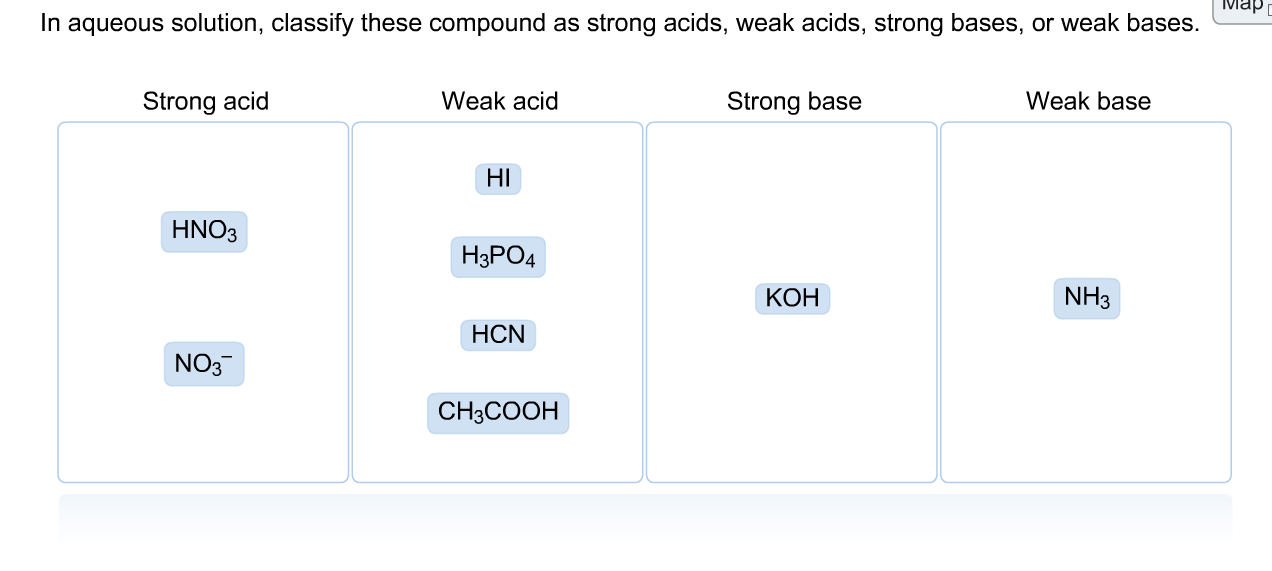
SOLVED: Classify these compounds by whether they behave as strong acids, weak acids, strong bases or weak bases in aqueous solution Strong acid Weak acid Strong base Weak base HCIO, HCN HS (

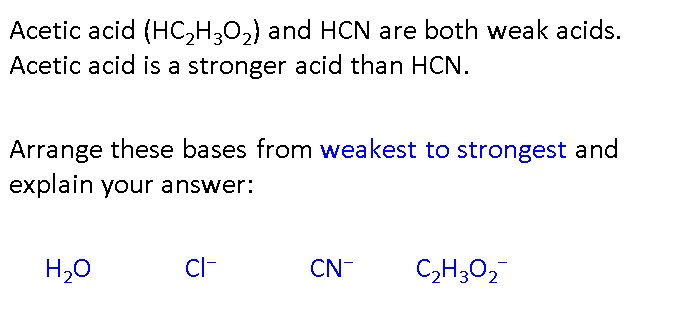
SOLVED: HCI (aq) is strong acid, which means that HCI is stronger acid that H;O . HF and HCN are weak acids, and HF is stronger than HCN. Rank the basic strength

Which should be stronger acid, HOCN, or HCN? Explain briefly In HOCN, the H+ ion is attached to th - YouTube

HCN is a weak acid ( Ka = 6.2 × 10^-10 ) ,NH4OH is a weak base ( Kb = 1.8 × 10^-5 ) . A 1.00 M solution of NH4CN would be:
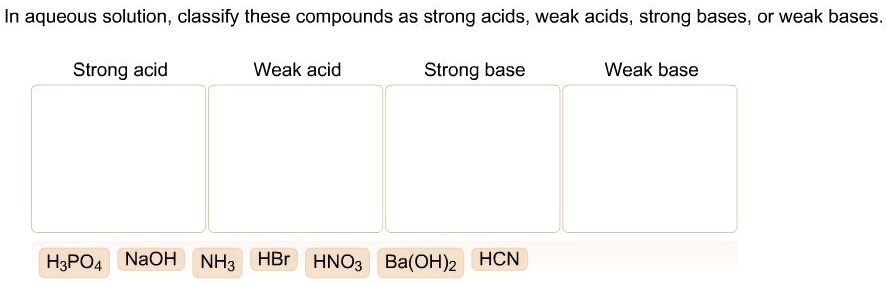
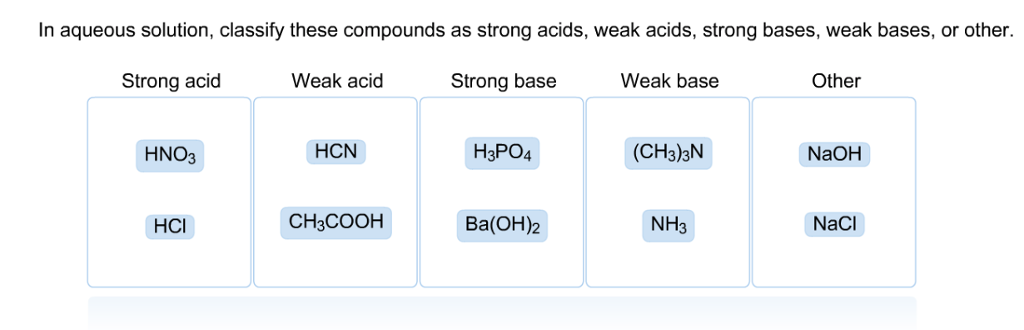
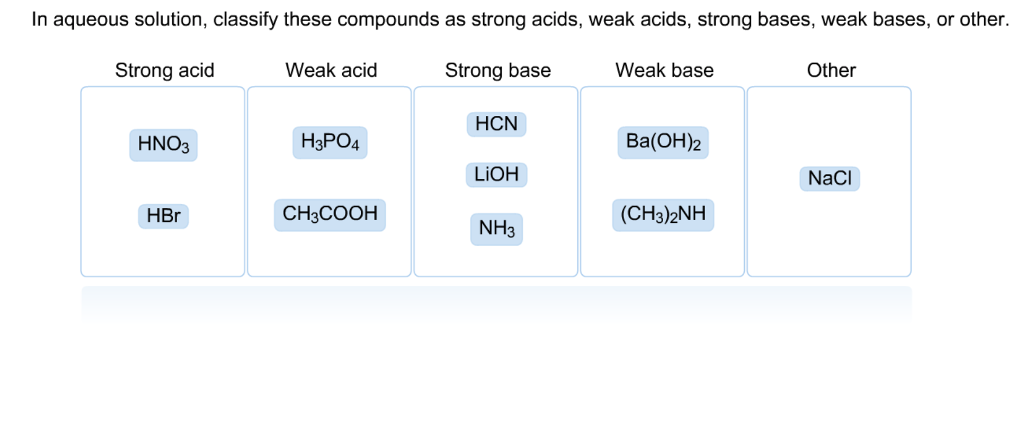
SOLVED: In aqueous solution, classify these compounds as strong acids, weak acids, strong bases or weak bases Strong acid Weak acid Strong base Weak base HaPO4 NaOH NH3 HBr HNO3 Ba(OH)z HCN
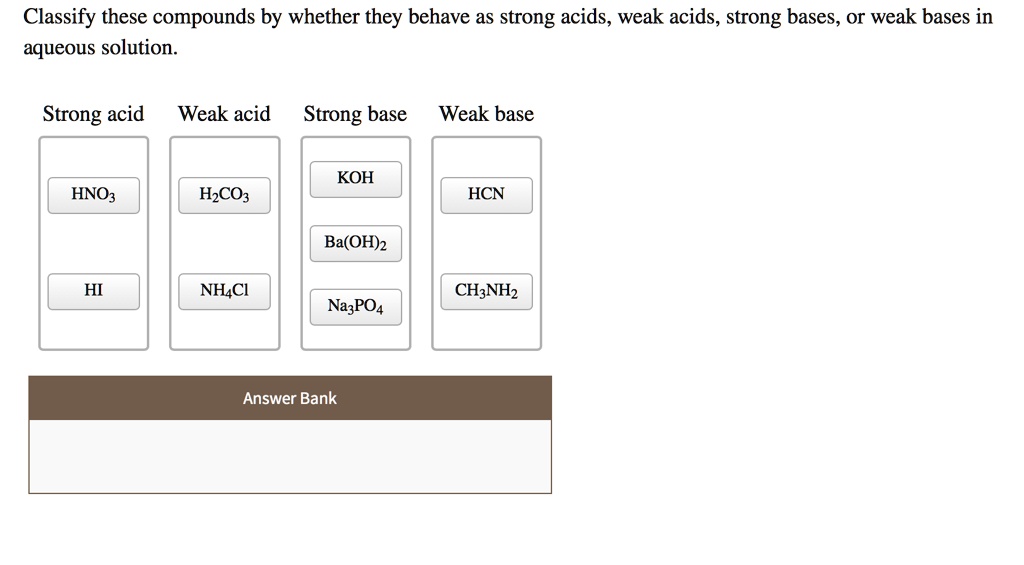
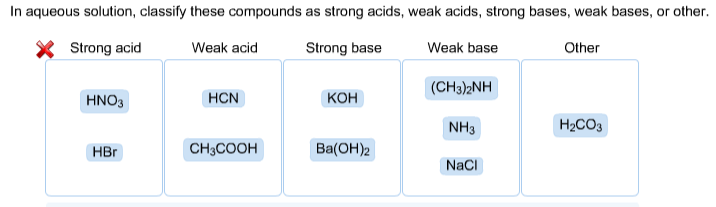
SOLVED: Classify these compounds by whether they behave as strong acids, weak acids, strong bases, Or weak bases in aqueous solution. Strong acid Weak acid Strong base Weak base KOH HNOz HzCOz