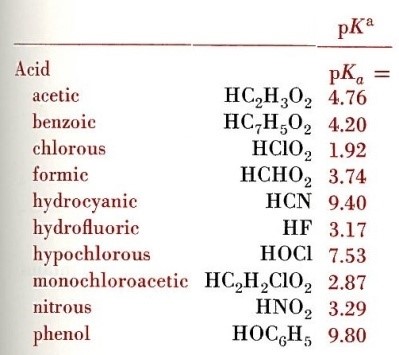
DFT and AIM Study of the Protonation of Nitrous Acid and the pKa of Nitrous Acidium Ion | The Journal of Physical Chemistry A

OneClass: The standard electrochemical potential for the reduction of nitrate ion (NO_3^- + 3H^+ + 2e...

PDF) DFT and AIM Study of the Protonation of Nitrous Acid and the p K a of Nitrous Acidium Ion | Ana Marulanda Rios - Academia.edu
What is the pH of a 0.40 M solution of sodium nitrite, NaNO2? The pKa for nitrous acid (HNO2) is 3.35 - Quora

SOLVED: 4J-1 113 The pH value ofa mixture Buffer of nitrous acid (HNOZ) Pka = 3.35 At a concentration of 0.12 M and sodium nitrite NaNO2 at a concentration of 0.15M Equal

The ionization constant of nitrous acid is 4.5 × 10^-4 . Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.

Microscale pH variations during drying of soils and desert biocrusts affect HONO and NH3 emissions | Nature Communications

Superoxide and Nitrous Acid Production from Nitrate Photolysis Is Enhanced by Dissolved Aliphatic Organic Matter | Environmental Science & Technology Letters

organic chemistry - Why Does A Brønsted–Lowry Acid Accept Proton from Stronger Acid? - Chemistry Stack Exchange



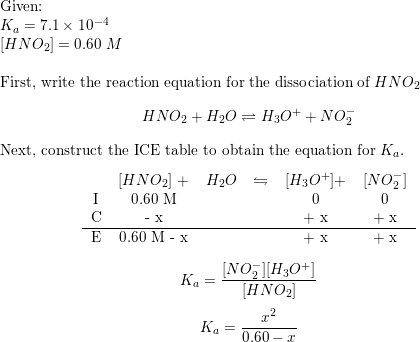





![ANSWERED] Use the pka table to select a base to depr... - Organic Chemistry ANSWERED] Use the pka table to select a base to depr... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/80475089-1659894957.2799208.jpeg)