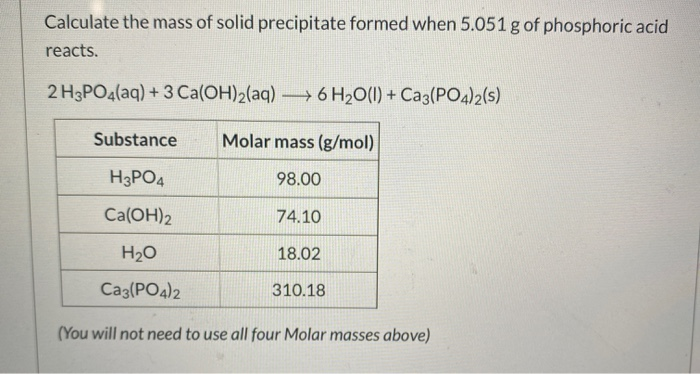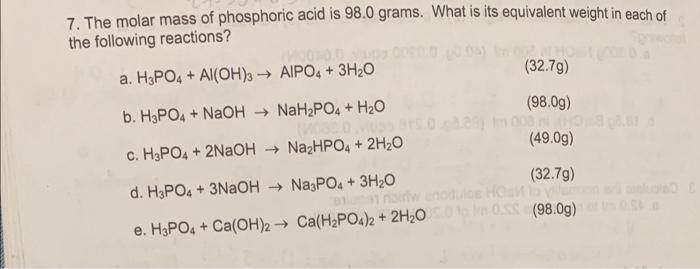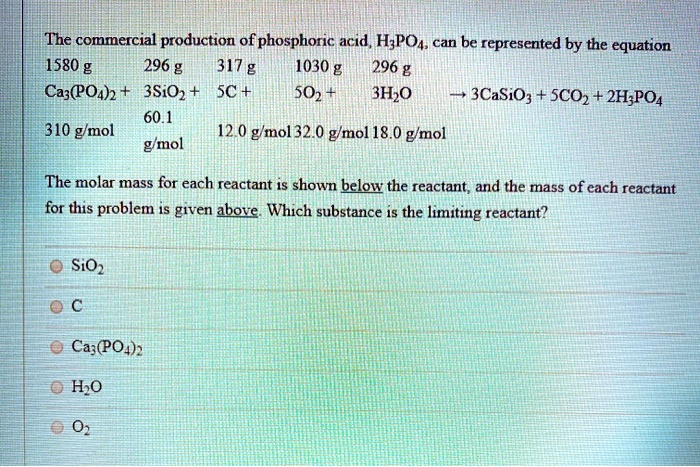
SOLVED: The commercial production of phosphoric acid, HzPOA can be represented by the equation 1580 g 296 g 317 g 1030 g 296 g Caz(PO4)2 3Si02 SC + 502 3HzO 3CaSiOg SCO2 +

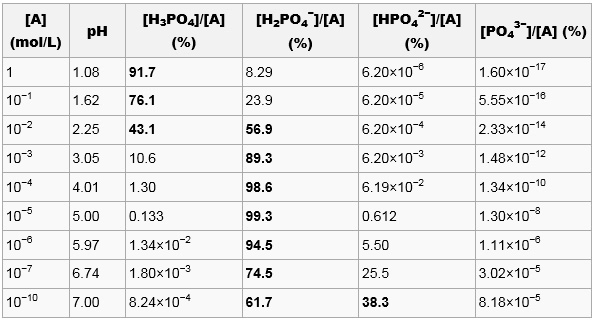
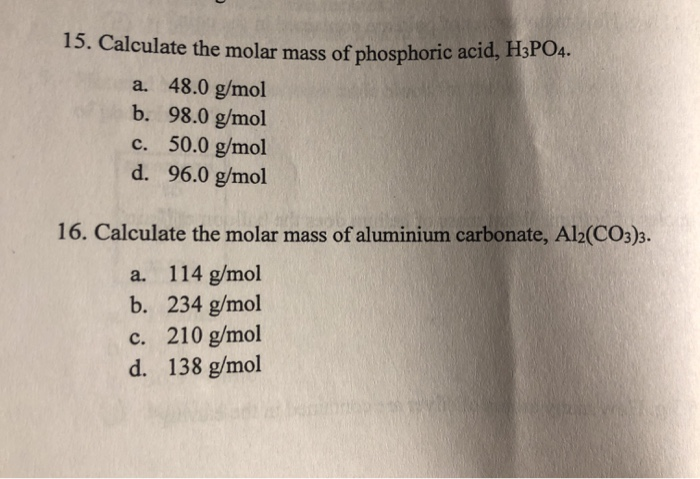
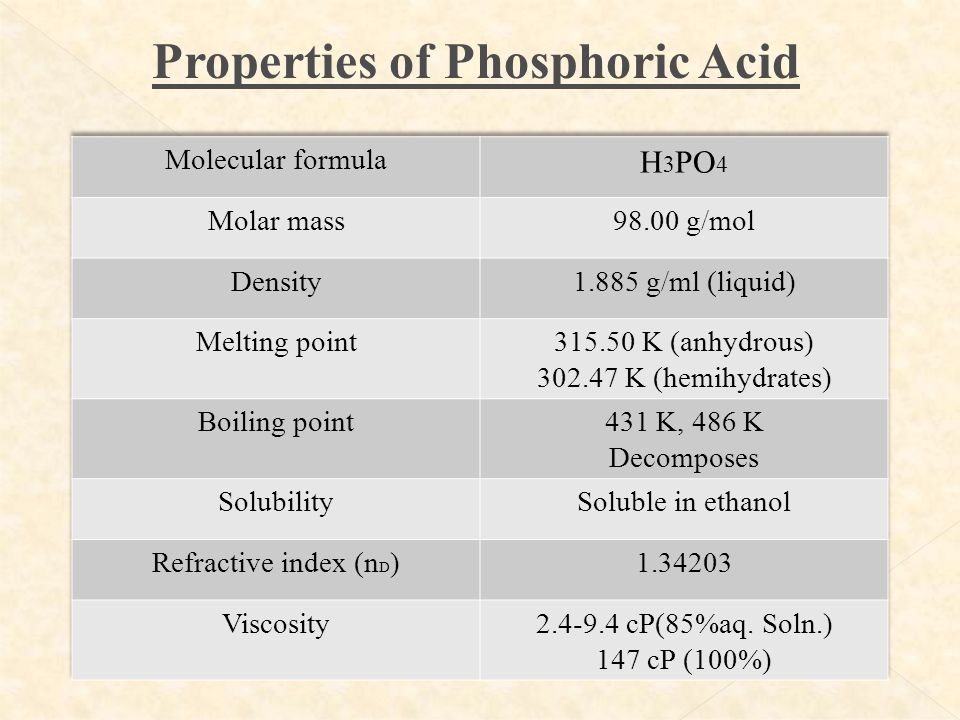
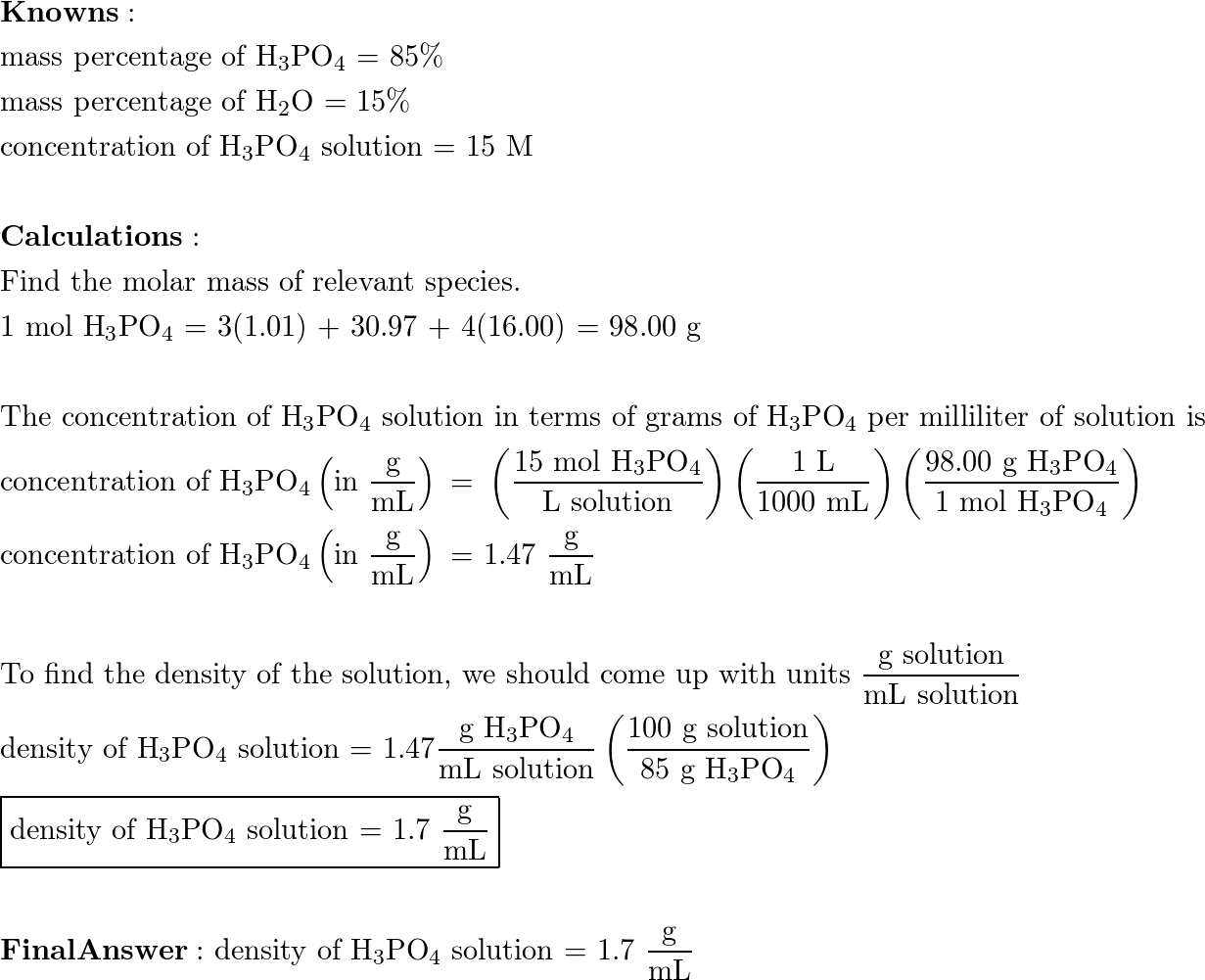
SOLVED: The morality of a 30.5% by mass aqueous solution of phosphoric acid H3PO4 is. molar mass of H3PO4 = 97.99g/mol

What is the equivalent weight of phosphoric acid `(H_(3) PO_(4))` according to the equation - YouTube
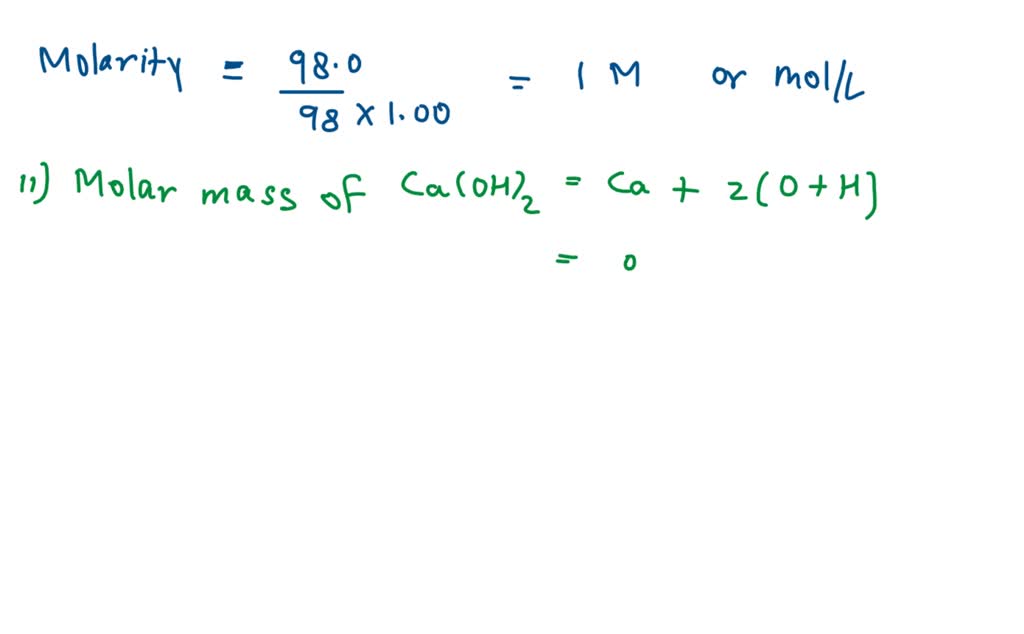
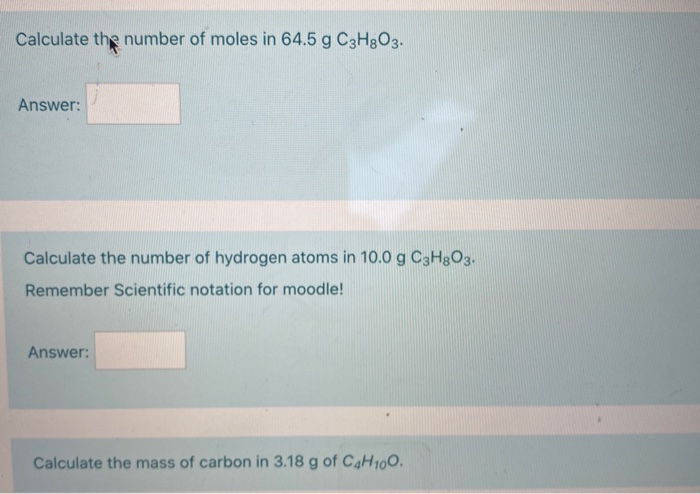
SOLVED: Find the molarity: 98.0 g of phosphoric acid, H3PO4, in 1.00 L of solution 0.2074 g of calcium hydroxide, Ca(OH)2, in 40.00 mL of solution 10.5 kg of Na2SO4·10H2O in 18.60 L of solution
![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.
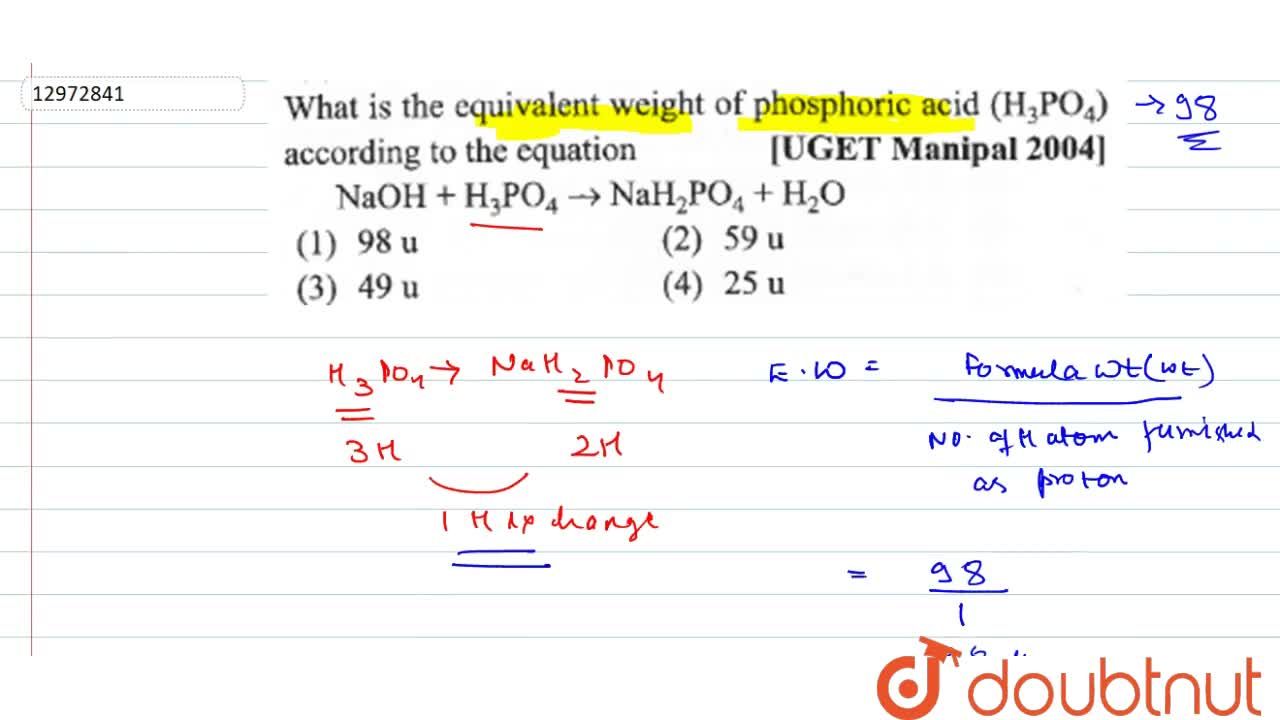
What is the equivalent weight of phosphoric acid (H(3) PO(4)) according to the equation NaOH + H(3) PO(4) rarr NaH(2) PO(4) + H(2)O