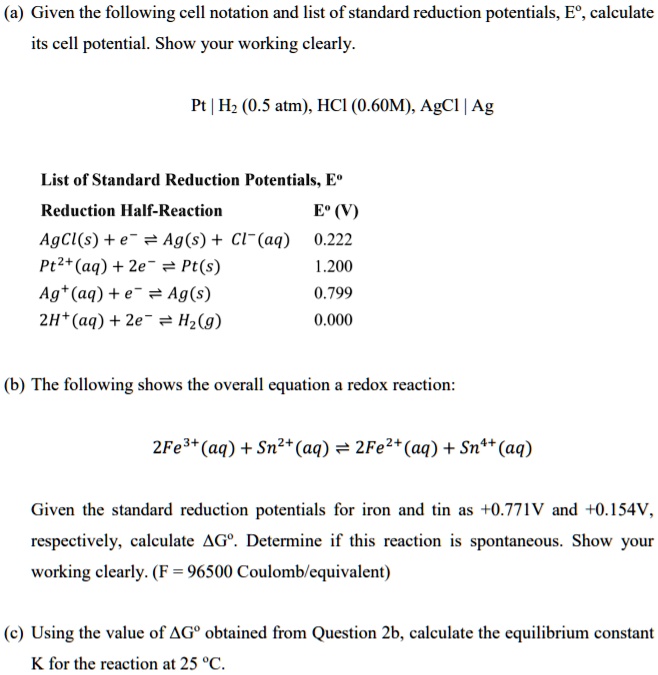
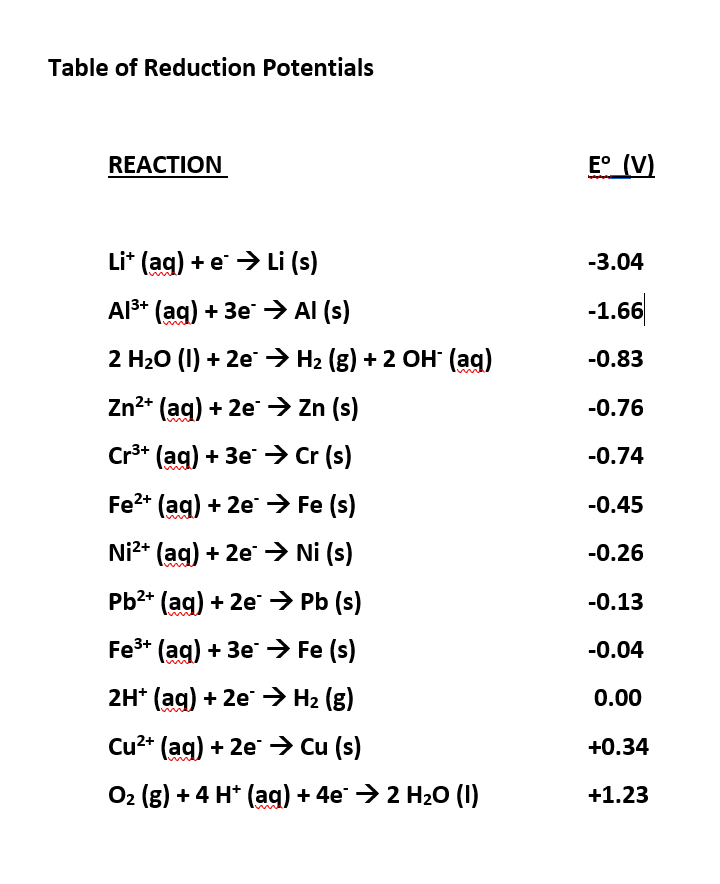
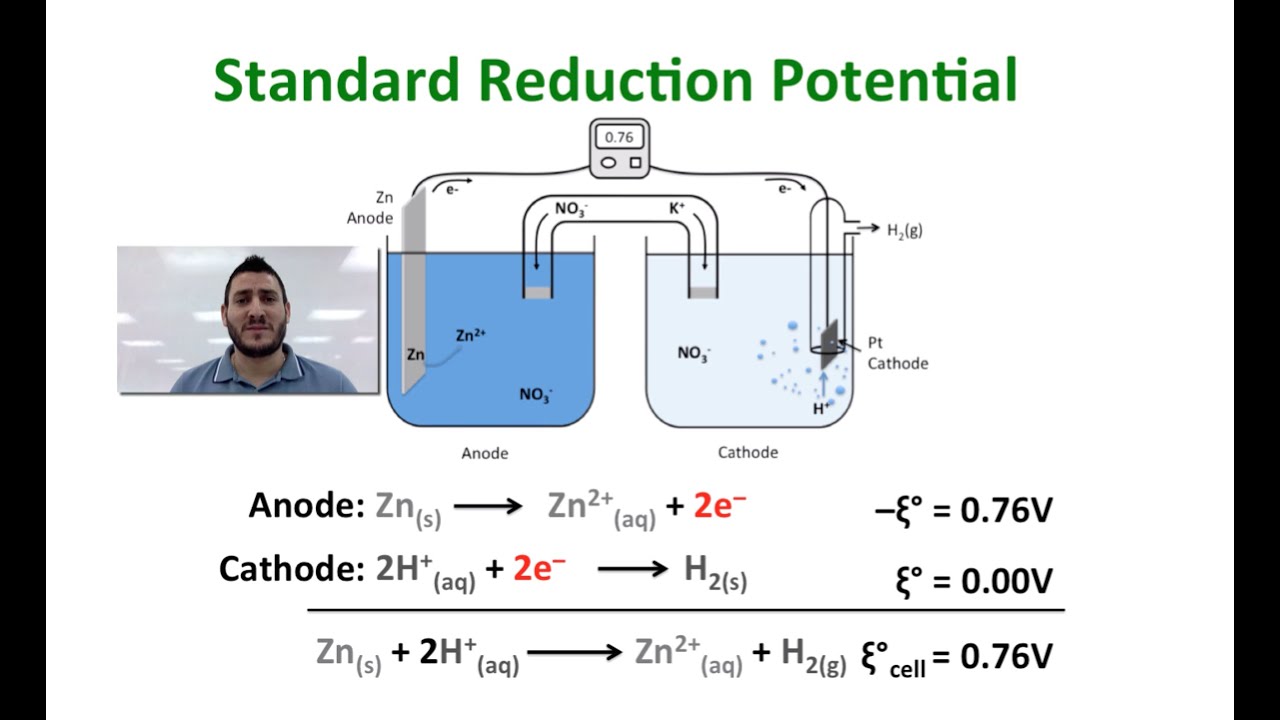
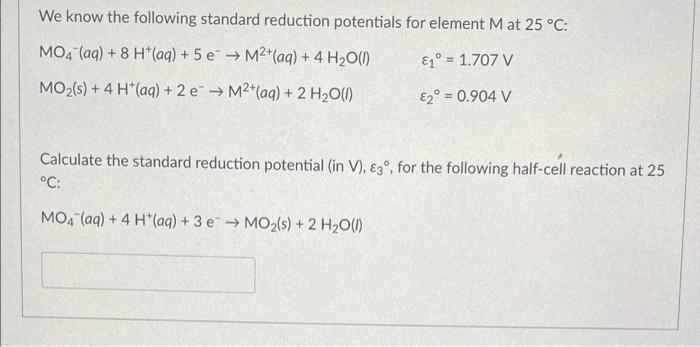
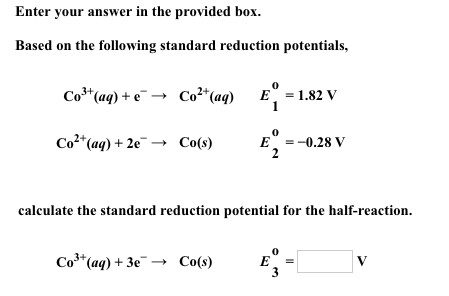
![SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of](https://cdn.numerade.com/ask_images/bd798f86fb6f4631925af95715b183c5.jpg)
SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .
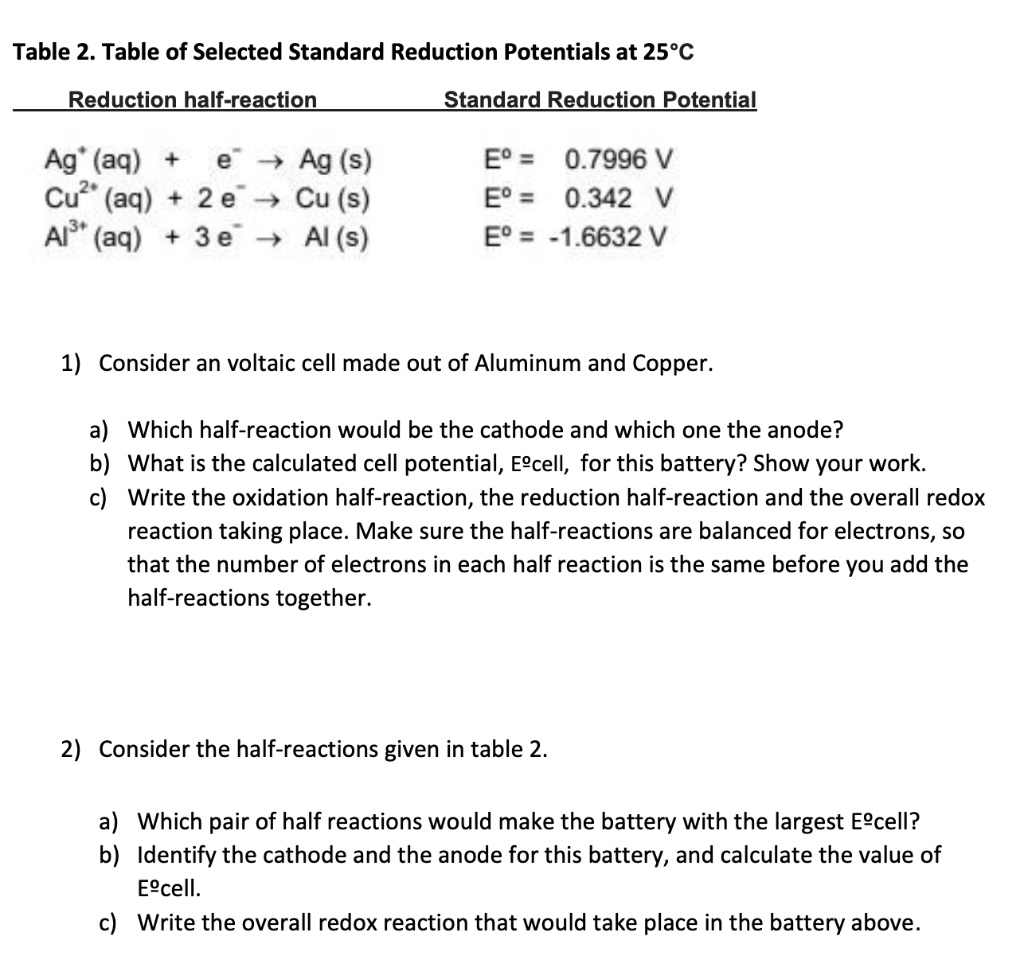
SOLVED: Table 2. Table of Selected Standard Reduction Potentials at 25*€ Reduction half-reaction Standard Reduction Potential Ag' (aq) e 4 Ag (s) Cu?" (aq) 2 e 4 Cu (s) Al"' (aq) 3
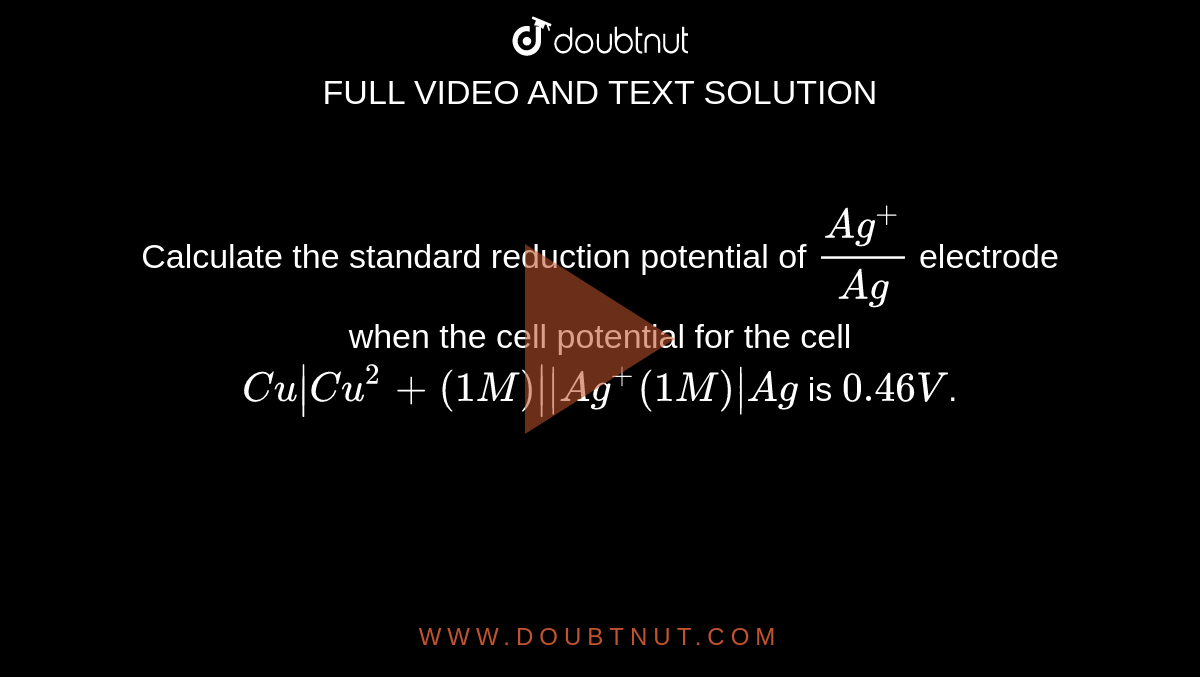
Calculate the standard reduction potential of (Ag^+)/ (Ag) electrode when the cell potential for the cell Cu | Cu^2+ (1M) | | Ag^+(1M) | Ag is 0.46 V.

Calculate the E and E^∘ of the cell Ni | Ni^2 + | | Cu^2 + | Cu from the following half - cell reactions: Ni^2 + + 2e^-→ Ni; E^∘ = -

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa