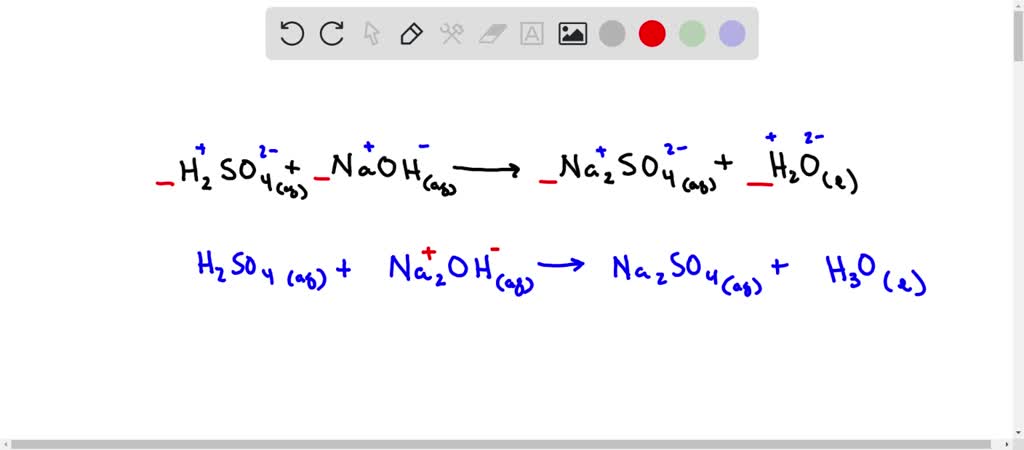
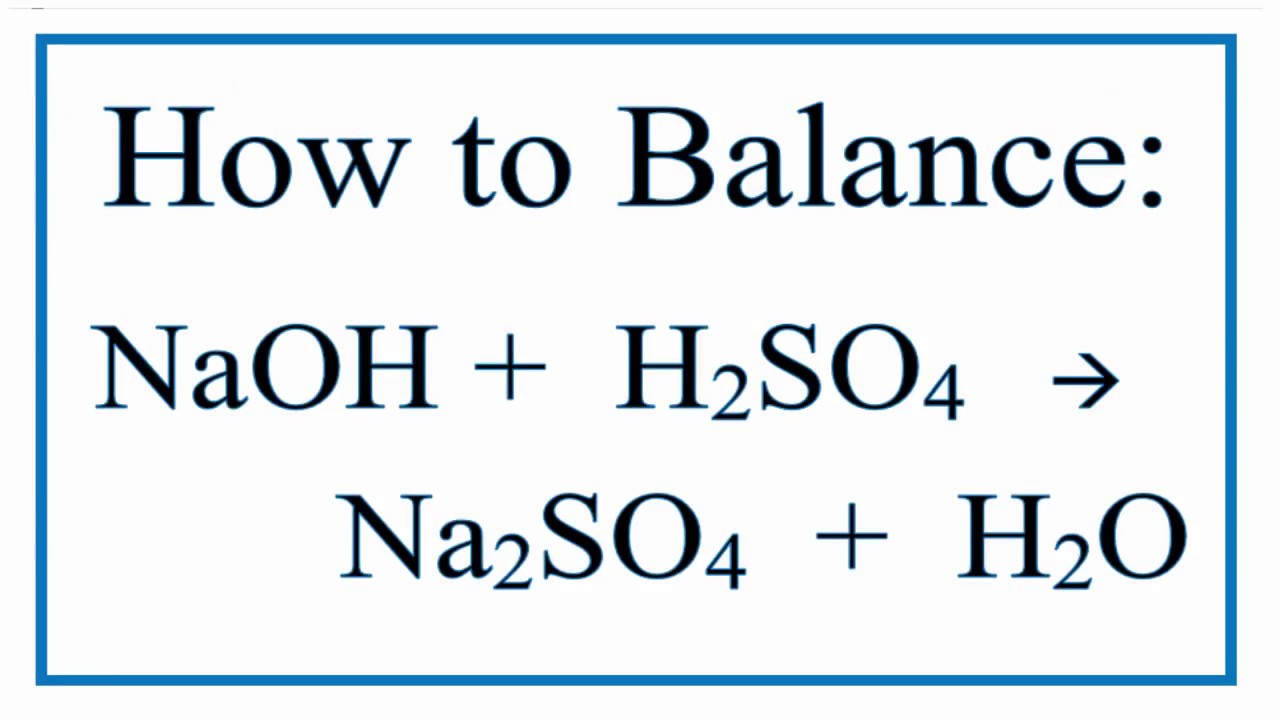
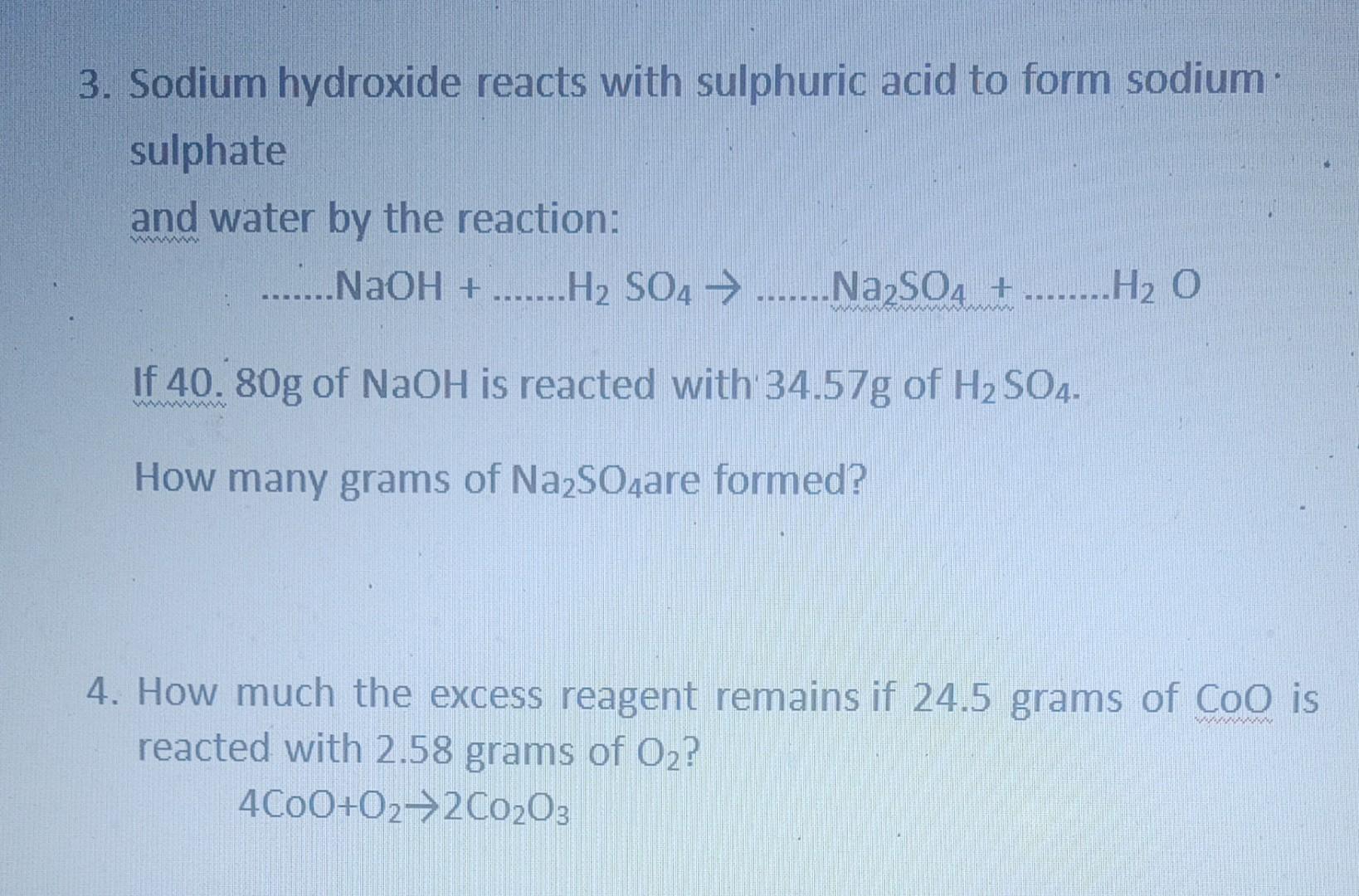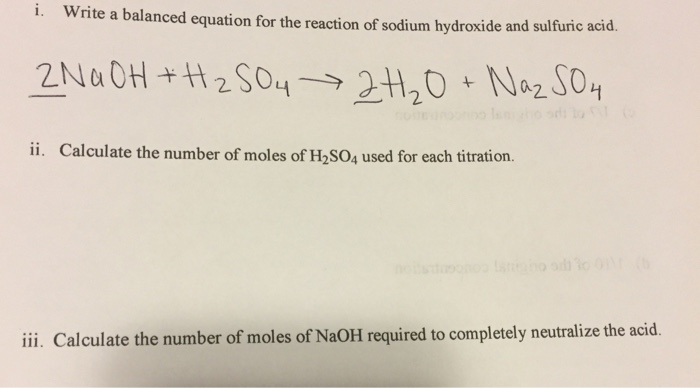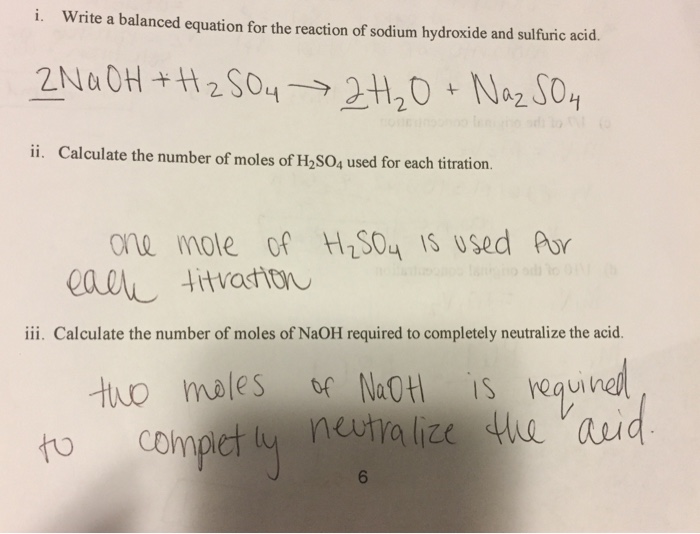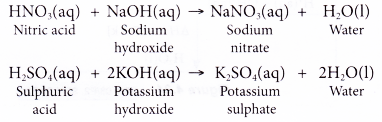Sulphuric acid reacts with sodium hydroxide as follows: H2SO4 + 2NaOH→ Na2SO4 + 2H2O When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of 0.1
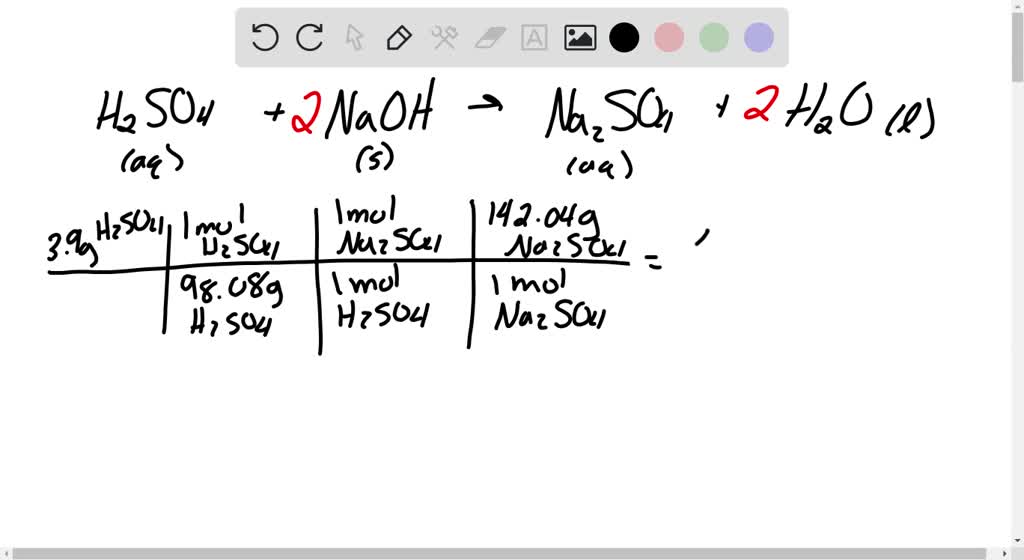
SOLVED: Aqueous sulfuric acid (H2SO4) reacts with solid sodium hydroxide ( NaOH) to produce aqueous sodium sulfate (Na2SO4) and liquid water (H2O). If 1.58 g of sodium sulfate is produced from the reaction
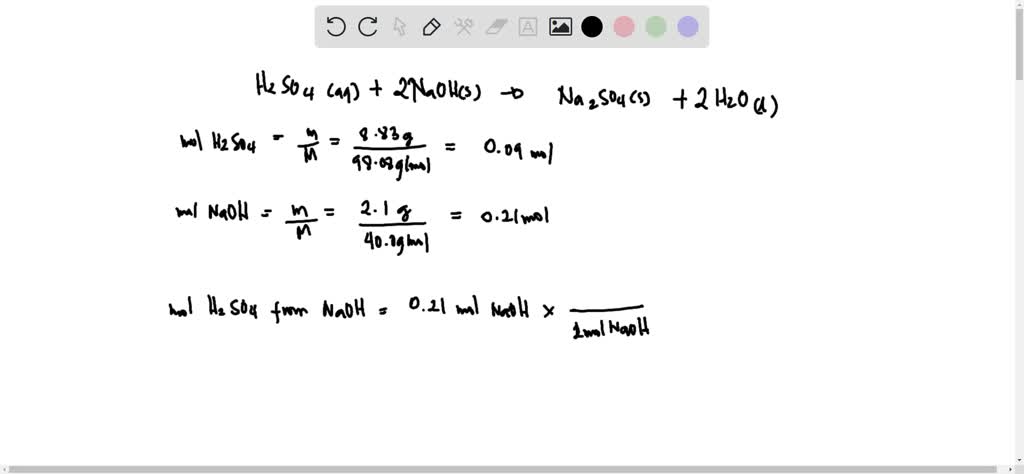
SOLVED: Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate Na2SO4 and liquid water H2O. Suppose 8.83 g of sulfuric acid is mixed with 2.1

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download

OneClass: Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous ...

Sulphuric acid reacts with sodium hydroxide as follows H(2)SO(4)+2NaOHrarrNa(2)SO(4)+2H(2)O when 1L of 0.1M sulphuric acid solution is allowed to react with 1L of 0.1M sodium hydroxide solution, the amount of sodium solphate

Sulphuric acid reacts with sodium hydroxide as follows H2SO4 + 2NaOH → Na2SO4 + 2H2O When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of

2.46 g of sodium hydroxide (molar mass = 40) are dissolved in water and the solution is made to 100 cm ^3 in a volumetric flask. Calculate the molarity (in mol/L) of the solution.